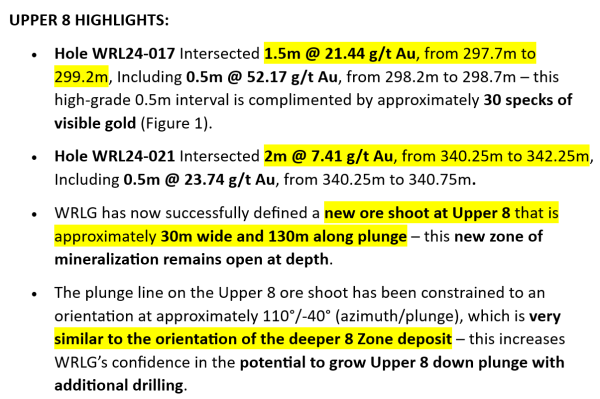
Better Therapeutics Follows Up Wins Including FDA Authorization For AspyreRx In 2023 With Securing FDA Breakthrough Device Designation Targeting Advanced Liver Disease
–News Direct–
By Faith Ashmore, Benzinga
Researchers, scientists and practitioners in the past few decades have begun to look at healthcare from a more holistic perspective, often challenging traditional medicine. The question on the minds of many is how to integrate holistic medicine into American healthcare at scale. One company, Better Therapeutics (NASDAQ: BTTX) is doing just that and trying to revolutionize how healthcare providers treat diseases like diabetes, metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH).
Better Therapeutics is taking a comprehensive approach to address the root causes of these diseases through the development of a novel form of cognitive behavioral therapy (CBT) that it delivers digitally enabling near-infinite scalability. Its novel form of CBT has been proven to work for the treatment of type 2 diabetes, helping patients make sustainable lifestyle changes by exploring how their thoughts and beliefs impact their actions and ultimately their health. However, like much of holistic medicine, without proper FDA authorization and established reimbursement pathways this type of treatment is not accessible or affordable for the millions of patients who could benefit from it.
Better Therapeutics has officially secured Breakthrough Device Designation for its groundbreaking CBT platform, intended to treat MASH. MASLD affects roughly 25%-30% of adults around the world, with an even higher prevalence of 75% among individuals with type 2 diabetes and up to 90% among those with advanced obesity. MASH, a more advanced form of the disease, affects approximately 20% of the 25% of American adults who have MASLD and is currently one of the leading indications for liver transplants.
Despite the growing rates of MASLD and MASH, there are currently no FDA-approved drugs or devices for treatment.
Milestones Hit In 2023
This news comes after Better Therapeutics LivVita study's results were published in the peer-reviewed journal Gastro Hep Advances, validating the CBT-based approach. The study successfully achieved its primary endpoint of reducing liver fat in just 90 days, along with significant improvements in liver health through key secondary endpoints, without any device-related adverse events. The study is the first of its kind to demonstrate improvements in various markers, such as FibroScan CAP score, MRI-PDFF, weight, ALT and FastTM score. These results suggest that the treatment has significant therapeutic potential for a larger patient population. Additionally, the study reported no device-related adverse events, even in patients with multiple comorbidities and background pharmacotherapy use.
In 2023, Better Therapeutics also received FDA Authorization for its first product, AspyreRxTM, to treat adults with type 2 diabetes. AspyreRx underwent rigorous clinical testing including a large randomized controlled trial to demonstrate safety and efficacy. Better Therapeutics reports that the trial showed statistically significant and clinically meaningful decreases in blood sugar when compared to a control group receiving the current standard of care. Specifically, over 50% of patients achieved a clinically meaningful response, with those patients experiencing an average drop of 1.3% in blood sugar as measured by HbA1c; this is similar to what is seen in other modern drug trials.
In addition, patients who used AspyreRx were also generally healthier: exploratory data revealed a range of cardiometabolic improvements, including blood pressure, body weight, quality of life, mood, safety and lower medication utilization compared to the control group. In September, the company completed enrollment in a real-world evidence program evaluating the long-term effectiveness of AspyreRx in type 2 diabetes.
Given the escalating prevalence, soaring costs and growing burden of cardiometabolic diseases, there is a crucial need to revolutionize how we approach their treatment. Better Therapeutics revolutionary approach could open doors for physicians and patients who are looking for more effective methods of treatment. While companies like Novo Nordisk (NYSE: NVO) and Eli Lilly (NYSE: LLY) have seen success with diabetes medication, Better Therapeutics transformative solution may have the potential to change the trajectory of the disease.
Benzinga is a leading financial media and data provider, known for delivering accurate, timely, and actionable financial information to empower investors and traders.
This post contains sponsored content. This content is for informational purposes only and is not intended to be investing advice.
Contact Details
Benzinga
+1 877-440-9464
Company Website
View source version on newsdirect.com: https://newsdirect.com/news/better-therapeutics-follows-up-wins-including-fda-authorization-for-aspyrerx-in-2023-with-securing-fda-breakthrough-device-designation-targeting-advanced-liver-disease-750818129
Benzinga
COMTEX_448374717/2655/2024-02-26T08:16:19
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No DFHS Newspaper journalist was involved in the writing and production of this article.